- 92 Uranium 235 Atomic Number
- Isotope Uranium 235 Atomic Number
- Uranium 235 Number Of Protons
- Atomic Structure Of Uranium 235
- Typically, when uranium 235 nucleus undergoes fission, the nucleus splits into two smaller nuclei (triple fission can also rarely occur), along with a few neutrons (the average is 2.43 neutrons per fission by thermal neutron) and release of energy in the form of heat and gamma rays. The average of the fragment atomic mass is about 118, but very.
- 1) When Uranium-235 (atomic number 92/mass number 235) is struck by a slow neutron it is split into two smaller elements and 3 neutrons (atomic number 0/mass number 1). If one of the elements it splits into is Strontium (Sr) (atomic number 38/mass number 90), what is the other element it splits into? Must include atomic number and mass number.
U = Atomic Mass = 235. Atomic Number = 92. Α-particle = Atomic Mass = 4. Atomic Number = 2. Subtract the α-particle from the uranium: 235 - 4 = 231 = Atomic Mass of decay product. 92 - 2 = 90 = Atomic Number of decay product. Element 90 is thorium, so the decay product is thorium-231.

Atomic Number of Uranium is 92.

Chemical symbol for Uranium is U. Number of protons in Uranium is 92. Atomic weight of Uranium is 238.02891 u or g/mol. Melting point of Uranium is 1132,4 °C and its the boiling point is 3818 °C.
» Boiling Point» Melting Point» Abundant» State at STP» Discovery YearAbout Uranium
Uranium is a highly radioactive silvery metal discovered in the 18th century and named after the planet Uranus. It is very toxic and is dangerous to all living organisms on this planet. Uranium can be extracted from a number of natural ores like carnonites, brannerites, and others. This element is a key one that made it possible to obtain nuclear energy and provide the humanity with it. Uranium-235 is used to initiate nuclear chain reactions and create huge amounts of heat which is used as a source of energy for people and their needs. On the other hand, it is used for developing nuclear weapons, nuclear submarines, and other things.
Properties of Uranium Element
| Atomic Number (Z) | 92 |
|---|---|
| Atomic Symbol | U |
| Group | |
| Period | 7 |
| Atomic Weight | 238.02891 u |
| Density | 18.95 g/cm3 |
| Melting Point (K) | 1405.3 K |
| Melting Point (℃) | 1132,4 °C |
| Boiling Point (K) | 4404 K |
| Boiling Point (℃) | 3818 °C |
| Heat Capacity | 0.116 J/g · K |
| Abundance | 2.7 mg/kg |
| State at STP | Solid |
| Occurrence | Primordial |
| Description | Actinide |
| Electronegativity (Pauling) χ | 1.38 |
| Ionization Energy (eV) | 6.19405 |
| Atomic Radius | 175pm |
| Covalent Radius | no datapm |
| Van der Waals Radius | 186 |
| Valence Electrons | 2 |
| Year of Discovery | 1789 |
| Discoverer | Klaproth |
What is the Boiling Point of Uranium?
Uranium boiling point is 3818 °C. Boiling point of Uranium in Kelvin is 4404 K.
What is the Melting Point of Uranium?
Uranium melting point is 1132,4 °C. Melting point of Uranium in Kelvin is 1405.3 K.
How Abundant is Uranium?
Abundant value of Uranium is 2.7 mg/kg.
92 Uranium 235 Atomic Number
What is the State of Uranium at Standard Temperature and Pressure (STP)?
Isotope Uranium 235 Atomic Number
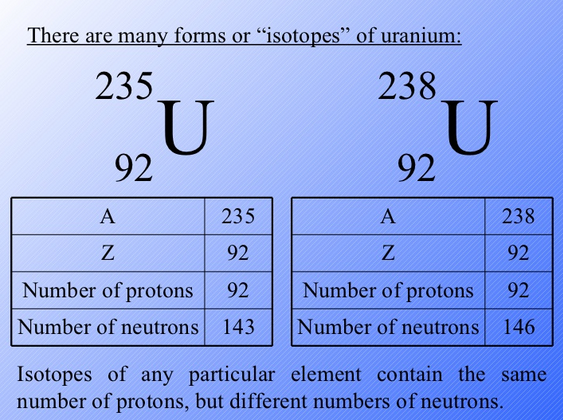
Uranium 235 Number Of Protons
State of Uranium is Solid at standard temperature and pressure at 0℃ and one atmosphere pressure.
When was Uranium Discovered?
Uranium was discovered in 1789.
Atomic Structure Of Uranium 235
