- Phosphorus Electrons And Protons
- Phosphorus Electrons Number
- Phosphorus Electrons Gained Or Lost
- Phosphorus Electrons How Many
- Phosphorus Electrons Needed To Become Stable
Lewis Structures
Lewis electron dot structures are representations of the distribution of electrons in molecules and ions. They are useful in determining the three-dimensional shape of a molecule or ion. A Lewis structure can be drawn for a molecule or ion by following three steps:
In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's nucleus.The closest shell to the nucleus is called the '1 shell' (also called the 'K shell'), followed by the '2 shell' (or 'L shell'), then the '3 shell' (or 'M shell'), and so on farther and farther from the nucleus. Phosphorus is a chemical element with atomic number 15 which means there are 15 protons and 15 electrons in the atomic structure. The chemical symbol for Phosphorus is P. As an element, phosphorus exists in two major forms—white phosphorus and red phosphorus—but because it is highly reactive, phosphorus is never found as a free element on Earth.

Step 1: Count the total number of valence electrons.
For a neutral molecule, sum the numbers of valence electrons of each atom in the molecule. For a negative ion, add to the sum the magnitude of the charge. For a positive ion, subtract from the sum the magnitude of the charge.
What is the total number of valence electrons for each of the following molecules or ions? | Click here to see Periodic Table |
Remember the valence electrons for each atom is the same as the A group number in the periodic table. Also remember that a negative charge will add to the valence electron count. Try again!
The correct answers have been entered for you. Make sure and review the calculation below!
| Molecule or Ion | Atoms | Valence Electrons | Total Valence Electrons |
| PH3 | P | 5 | 8 |
| 3 H | 3 x 1 | ||
| CF4 | C | 4 | 32 |
| 4 F | 4 x 7 | ||
| NO3- | N | 5 | 24 |
| 3 O | 3 x 6 | ||
| -1 charge | 1 |
Step 2: Decide on the arrangement of atoms.
The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds. If all of the atoms usually form the same number of bonds, the least electronegative atom is usually the central atom.
Click on the atom in each of the molecules or ions below that will be the central atom.
| PH3 | CF4 | NO3- |
Fluorine can only make one bond! Try again.
Which atom in the formula has the smallest subscript? Try again!
Step 3: Arrange electrons around the atoms so that each atom has an octet.
EXCEPTIONS TO THE OCTET RULE:
- Hydrogen will only have two electrons.
- Group 3A (boron, aluminum, etc.) may only have six electrons.
- Atoms in the third row and beyond may expand their octet (have more than eight electrons) if needed.
Clicking on an atom in the structures below will add a lone pair of electrons. Clicking on a bond will add a pair of electrons to the bond (making a single bond a double bond). Arrange electrons around the atoms in each structure so each atom has an octet. The number of valence electrons for each molecule or ion is shown beneath the structure.
|
|
| |||||||||||||||||||||||||||||||
Remember hydrogen will not have more than two electrons. This hydrogen is part of a covalent bond (sharing two electrons).

A double bond here would cause hydrogen to share four electrons with phosphorus. Remember that hydrogen will not have more than two electrons.
You better count the electrons already included in your Lewis structure! Remember this structure should only have eight electrons.
Good! This Lewis structure has eight electrons - one lone pair on phosphorus (2) and three bonds (6). The phosphorus has eight electrons, and each hydrogen has two electrons.
This structure should only have eight electrons! The three bonds phosphorus makes to the hydrogen atoms account for six electrons. Where should you place the remaining two electrons?
This fluorine already has eight electrons (an octet) - three lone electron pairs (6 electrons) and one bond (2 electrons).
Carbon is making four bonds (8 electrons) - it already has an octet!
Putting another bond here would definately cause carbon to have more than eight electrons. You better try something else.
Each atom in this Lewis structure should have an octet of electrons (8 electrons). Remember that each lone electron pair counts as two electrons and each bond counts as two electrons (for each of the atoms participating in the bond).
Good! Each atom in this Lewis structure has an octet and the structure has a total of 32 electrons.
Putting another lone electron pair on this oxygen will cause it to have greater than eight electrons.
Phosphorus Electrons And Protons
This nitrogen already as eight electrons (one lone pair and three bonds).
Putting another bond here would cause nitrogen to have more than eight electrons. You should try something else.
Phosphorus Electrons Number
Good! This Lewis structure has a total of 24 electrons and each atom has an octet. A complete Lewis structure for an ion is bracketed and includes the charge.
While each atom in this structure has an octet, you have used too many electrons! This ion only has 24 electrons.
Phosphorus Electrons Gained Or Lost
The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. The outer shell arrangement therefore resembles that of nitrogen, with three half-filled orbitals each capable of forming a single covalent bond and an additional lone-pair of electrons. Depending on the electronegativity of the elements with which it combines, phosphorus can therefore exhibit oxidation states of +3 or −3, just as does nitrogen. The principal differences between nitrogen and phosphorus are that the latter is of considerably lower electronegativity and has larger atoms, with outer d orbitals available. For these reasons, the similarities between nitrogen and phosphorus chemistry are largely formal ones, tending to conceal the actual, wide differences. The outer d orbitals in phosphorus permit an expansion of the octet, which leads to the +5 state, with five actual covalent bonds being formed in compounds, a condition impossible for nitrogen to achieve.
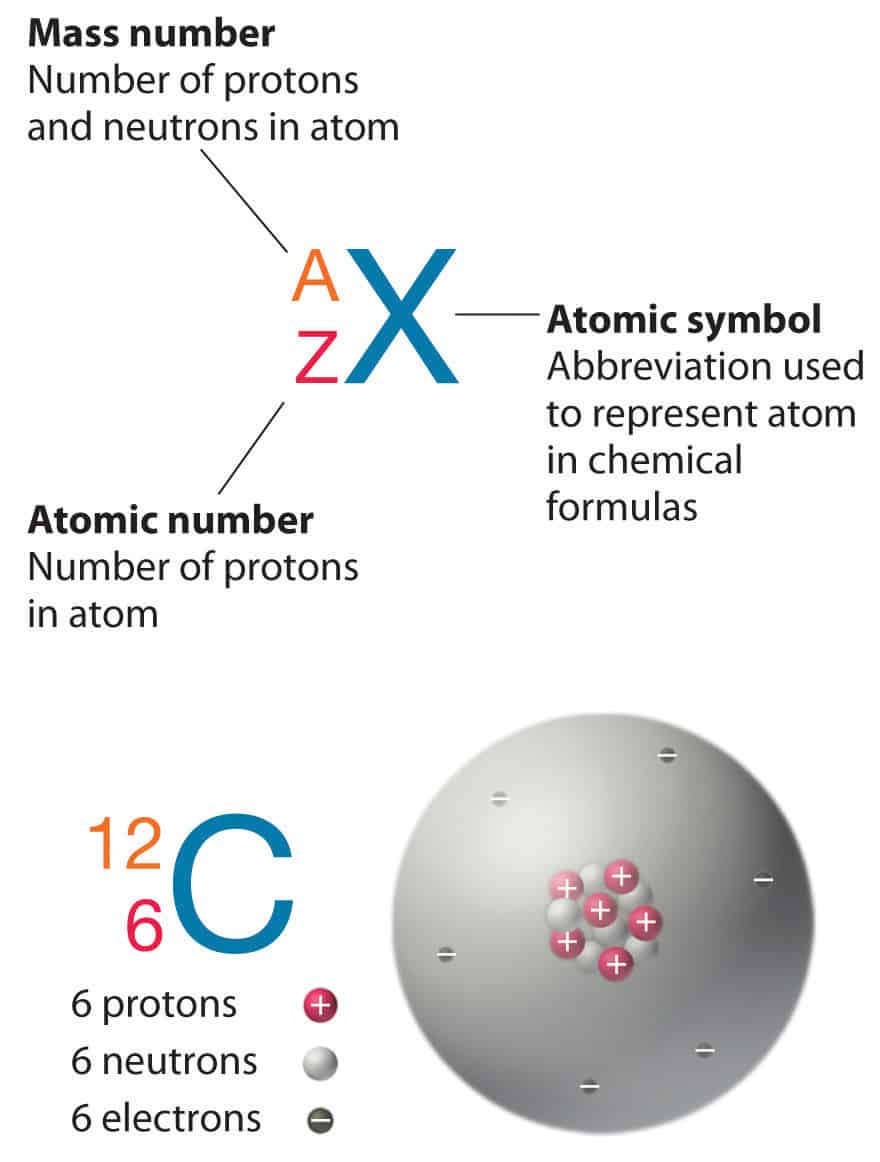
Phosphorus Electrons How Many
The first striking difference in chemistry of the two elements is that elemental phosphorus exists under ordinary conditions in any of 10 modifications, or allotropic forms, all of which are solid; the three major allotropes are white, red, and black. Phosphorus molecules of formula P2, structurally analogous to N2 molecules and evidently also triply bonded, exist only at very high temperatures. These P2 molecules do not persist at lower temperatures—below about 1,200 °C (2,200 °F)—because of the fact that three single bonds in phosphorus, in contrast to the situation with nitrogen, are energetically favoured over one triple bond. On cooling, the triply bonded P2 molecules condense to form tetrahedral P4 molecules, in which each atom is joined to three others by single bonds. White phosphorus has two allotropes: the alpha form, which is stable at ordinary temperatures, has a cubic crystal structure; the beta form, which is stable below −78 °C (−108 °F), has a hexagonal crystal structure. Because of the relatively weak intermolecular attractions (van der Waals forces) between the separate P4 molecules, the solid melts easily at 44.1 °C (111.4 °F) and boils at about 280 °C (536 °F). Formation of tetrahedra requires bond angles of 60° instead of the preferred 90°–109° angles, so that white phosphorus is a relatively unstable, or metastable, form. It changes spontaneously, but slowly, at temperatures around 200 °C (390 °F) or higher, to a polymeric form called “red phosphorus.” This substance is amorphous when formed at lower temperatures, but it can become crystalline, with a melting point of about 590° C (1,090 °F). At higher temperatures and pressures, or with the aid of a catalyst, at ordinary pressures and a temperature of about 200 °C, phosphorus is converted to a flaky black crystalline form, which somewhat resembles graphite. This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation. In both the red and the black forms, each phosphorus atom forms three single bonds, which are spread apart sufficiently to be relatively strain free.
Phosphorus Electrons Needed To Become Stable
Consistent with the metastable condition of the white modification, and the crowding of its covalent bonds, this form is far more reactive chemically than the others. It is highly toxic, reacts vigorously with most reagents, and inflames in air at only 35° C (95 °F), so it must be stored under water or other inert liquid. White phosphorus dissolves readily in solvents such as carbon disulfide, in which it maintains the composition P4. White phosphorus has been used for military purposes as a source of smoke and to fill incendiary shells and grenades. In contrast, red phosphorus is insoluble and relatively inert, although large quantities of the usual commercial form can ignite spontaneously in air and react with water to form phosphine and phosphorus oxyacids. Red phosphorus is used in preparing the striking surface for safety matches. Black phosphorus is more inert and is capable of conducting electricity. Both these polymeric forms are insoluble and are very much less volatile than white phosphorus.
